How to create a Quality Management System in SharePoint Online
One of the common requirements, especially for Engineering and Manufacturing firms, is creating a Quality Management System (QMS). There are, of course, dedicated off-the-shelf software solutions already available on the market. However, with the wide adoption of SharePoint and Teams, the obvious question is whether or not applications and features available within the Microsoft 365 suite can be used to satisfy QMS requirements. So in this article, I would like to share my experience and explain how SharePoint, along with some other apps, can be used to build QMS functionality.
First, I want to state that I am not an expert in Quality or Quality standards or QMS in general. My primary expertise is SharePoint. With that said, I built many QMS sites for clients, to satisfy requirements like ISO 9001 or similar standards.
Quality is rather a vast area that encompasses many different requirements and objectives. So I thought the best way to structure this article would be to break Quality Management System into various areas and explain how a certain application or piece of functionality can serve to satisfy a certain request. Depending on your needs, you might need to satisfy all or just some of the requirements below.
Requirement 1: A place to store Policies, Procedures, SOPs, Work Instructions
SharePoint is an awesome match for this requirement, with its vast list of document management features. At a minimum, this is achieved via the out-of-the-box document libraries with built-in Version History, Check Out feature. A great addition to these requirements is the metadata feature, which will allow you to categorize your Policies, Procedures, Work Instructions, via metadata (i.e., Status, Expiration Date, etc.)
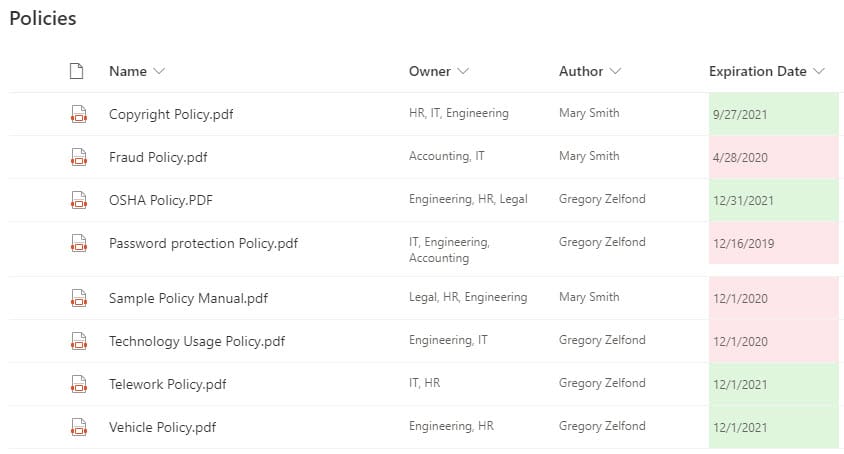
Requirement 2: Ability to organize Audit information
Another core requirement of many QMS systems is the ability to manage Audits. This can either be done with lists or, even better, Document Sets. The idea is that you would create a document library, configure it with document sets, and add some Audit (Doc Set) metadata to it (i.e., Audit #, Status, Date, etc.). This way, you would have a folder for each audit with corresponding files underneath.
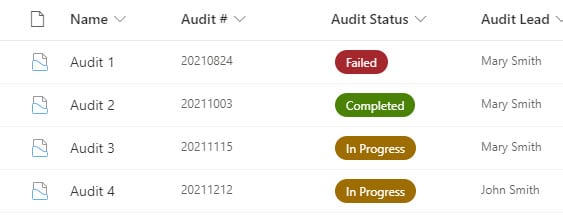
Requirement 3: Audit Trail of information
With QMS, you always need to have some traceability for document updates and revisions; this is where SharePoint’s Version History shines! Versioning is enabled in SharePoint by default, and you can also utilize the power of both major and minor versions.
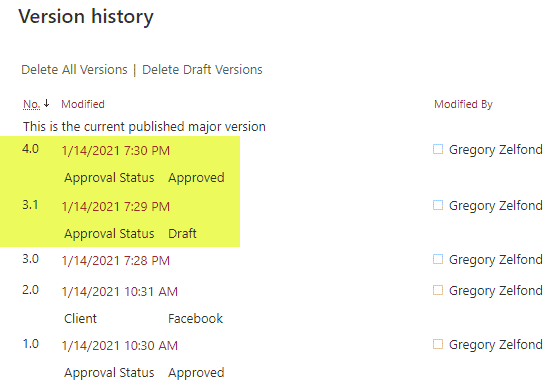
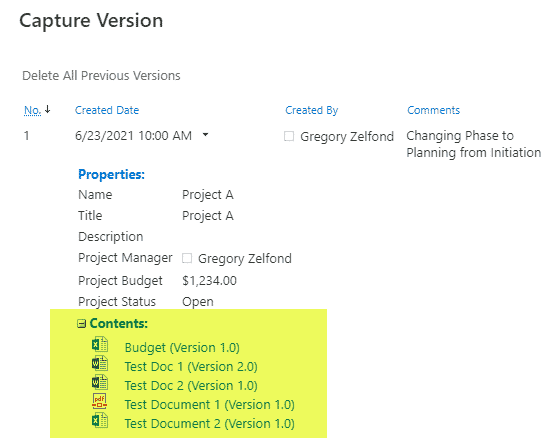
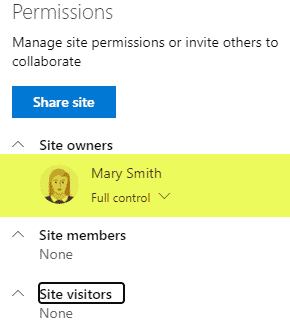
Requirement 4: Security
Security is yet another important aspect of QMS. Some information should be kept private and off-limits to the rest of the organization, while some information will be shared with the rest of the organization. Some users might have the ability to add/edit/delete, while others will only have read-only privileges. The good news is that SharePoint allows for quite customized security/permissions setup with various levels where you can set up security (site, doc library, folder, document) and custom permission levels you can create.
Requirement 5: CAPA (Corrective and Preventive Action)
For CAPA, I usually recommend a mix of MS Forms for deficiency submissions and a custom list for their tracking. You can customize both with your own metadata, views, format with different colors as necessary. In addition, you can use Power Automate to tie both together.
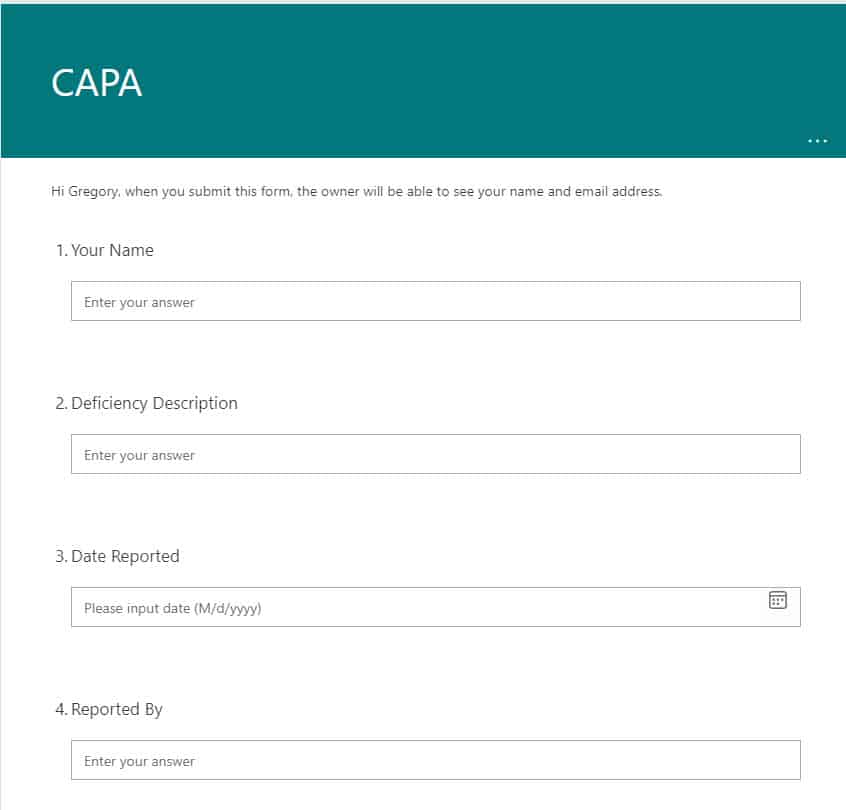
An example of MS Form used for CAPA submissions
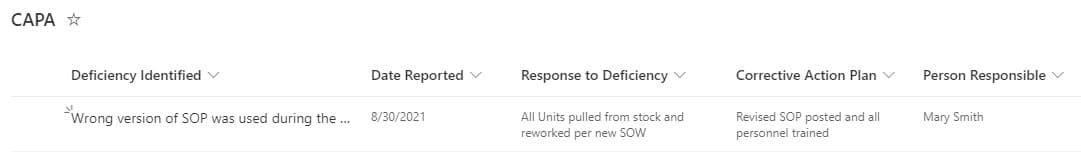
An example of a Custom List used for CAPA tracking
Requirement 6: Ability to display Process Diagrams, corresponding documents, work instructions, etc.
You often need to display process diagrams, along with related work instructions, procedures, and SOPs. For this, Site Pages are a perfect fit. You can create a Page to embed a diagram and other related documents. In addition, you can create a Page Template to make your knowledge base consistent. Finally, you can also add metadata to pages as well if need. The sky is the limit!
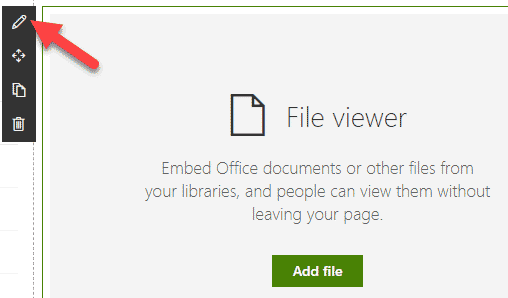
Requirement 7: Document Acknowledgement
Sometimes, you may require users to acknowledge they read or familiarized themselves with a certain policy or latest updater to a procedure or SOPs. Once again, Power Automate to the rescue. You can create a workflow in Power Automate and log the responses in a custom list or Excel.
Requirement 8: Ability to capture Customer Feedback
The core attribute that will define and drive continuous improvement in any quality management system is customer feedback. As a result, it might be necessary to easily collect and analyze such feedback. As far as collecting data from customers, you can easily do so via MS Forms. Information submitted can then be copied to custom lists stored in a SharePoint site. If you want to impress your leadership, you can then analyze the data via Power BI by building custom reports and dashboards.
Requirement 9: Ability to track review/expiration dates
One other important requirement of QMS is the ability to track expiration dates of policies and procedures. This is where SharePoint shines, with its ability to have a document library with metadata. On top of that, you can add conditional formatting (color coding) and workflow to automate notification to the proper recipients.

Requirement 10: Communication
Quality Management is not just about tracking policies and expiration dates. The big part of the Quality Management System is the ability to communicate information with the rest of the organization. This is where the SharePoint “Intranet” side of things shines. You can always build a communication site to share relevant QMS information with the organization and then utilize the various communication channels (Email, Yammer, Teams) for broadcasting the above information.
By the way, if you are looking for inspiration on what a Quality Site could look like in SharePoint, check out screenshots on my LookBook 365 portfolio site.
